INTRODUCTION TO CARBOHYDRATES
⇒ Carbohydrates are the polyhydroxy Aldehydes or Ketones, are major macronutrient and the primary sources of energy.
⇒ Consists of Carbon, Hydrogen, and Oxygen.
⇒ The general formula for carbohydrates is Cn(H2O)n.
⇒ For e.g. – C6(H2O)6 = C6H12O6
(n=6) (Glucose)
⇒ The sugars which contain Aldehydic group are called as Aldoses & the sugars which contain Ketonic group is called as Ketoses.
FUNCTIONS OF CARBOHYDRATES
⇒ Carbohydrates are the main source of energy.
⇒ 1 gm carbohydrates provides 3.9 Kcal
⇒ Carbohydrates are stored in our body in the form of Glycogen.
⇒ Excess of Carbohydrates converts into fats and stored in Adipose tissues.
⇒ Glycoprotein & Glycolipids are the components of cell membrane and receptors.
⇒ They are the components of Nucleic acids (DNA & RNA).
⇒ Carbohydrates are the structural bases of many organisms. For e.g. – Cellulose in plants & exoskeleton of insects.
⇒ The recommended dietary allowance for carbohydrates is 400gm/ day.
CLASSIFICATION OF CARBOHYDRATES
⇒ Carbohydrates have been broadly classified into three groups –
1.) Monosaccharides – They are the simple sugars consists of 3 – 7 carbon atoms.
NO. OF CARBON ATOMS ALDOSES KETOSES
TRIOSE (3-C) Glyceraldehyde Dihydroxyacetone
TETROSE (4-C) Erythrose Erythrulose
PENTOSE (5-C) Ribose Ribulose
HEXOSE (6-C) Glucose Fructose
HEPTOSE (7-C) Glucoheptose Sedoheptose
2.) Oligosaccharides – These are the compound sugars which consist of the 2–10 monosaccharides. If an oligosaccharide consists of two monosaccharides, it is called as disaccharides & the one which consists of three monosaccharides, called Trisaccharides & so on….
DISACCHARIDES MOLECULES PRESENT
Maltose Glucose + Glucose
Lactose Glucose + Galactose
Sucrose Glucose + Fructose
TRISACCHARIDES MOLECULES PRESENT
Raffinose Glucose + Fructose + Galactose
TETRASACCHARIDES MOLECULES PRESENT
Stachyose Glucose + Fructose + Galactose + Galactose
3.) Polysaccharides – These are the compound sugars which consist of more than 10 molecules of monosaccharides. If polysaccharides consist of the same type of monosaccharides, it is called as Homopolysaccharides & if the polysaccharide consists of different monosaccharides they are called as Heteropolysaccharides.
HOMO -
POLYSACCHARIDESHETRO -
POLYSACCHARIDES
Starch Hyaluronic acid
Glycogen Chondratin sulfate
Cellulose Dermatan sulfate
Chitin
ISOMERISM IN CARBOHYDRATES
⇒ Isomers are the compounds having same molecular formula but different structural formula and this phenomenon are known as isomerism.
⇒ Isomers are of two types –
- Structural isomers – The isomers having same molecular formula but different structure are called as structural isomers.
- Stereoisomers – The compounds having same molecular formula and structure but differs only in the spatial configuration.
⇒ Enantiomers – They are non-superimposed mirror images of each other. D- and L- isomers are mirror images of each other. When the hydroxyl group is present on the right side the sugar is D- isomer and if it is on the left side it is L- isomer. For e.g. D-Glyceraldehyde and L- Glyceraldehyde.

⇒ Diastereomers – The stereoisomers having the different configuration at two or more stereocenters are not the mirror images of each other they are called as diastereomers.

⇒ Optical activity – The substances which rotate the plane polarised light either towards right or towards left are said to be optically active substances and the property is known as optical activity. Those compounds which rotate light towards the right are called as Dextro-rotatory and the compounds which rotate plane polarised light towards left are known as Laevo-rotatory.
MONOSACCHARIDE STRUCTURES – FISCHER PROJECTION FORMULA or OPEN CHAIN STRUCTURES
1.) ALDOSES
I.) 3-C COMPOUND
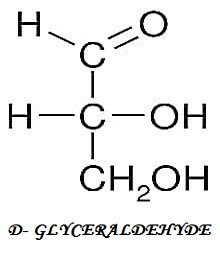
II.) 4-C COMPOUND
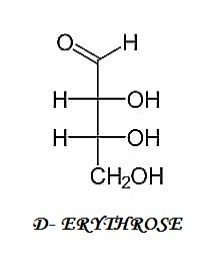

III.) 5-C COMPOUNDS
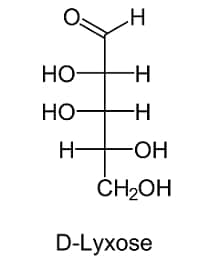
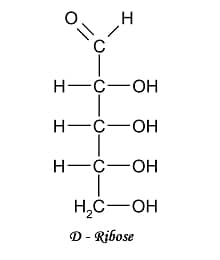


IV.) 6-C COMPOUND





CHEMICAL PROPERTIES OF CARBOHYDRATES
I.) Tautomerization or Enolisation
⇒ Tautomerization or Enolization is also known as Lobry De Bruyn Alberta’ van ekenstein transformation.
⇒ It is a type of isomerization in which glucose Fructose and mannose are interconvertible in weak alkaline solutions such as sodium hydroxide solution at low temperature.
II.) Reducing property of carbohydrates
⇒ Carbohydrates maybe reducing or nonreducing depending on reducing property of Carbohydrates because of free aldehyde or Ketone group of anomeric carbon.
⇒ Metal hydroxides like Copper Hydroxide oxidized the free aldehyde or Ketone group of reducing sugar.
III.) Oxidation reaction
⇒ The Terminal aldehyde group order terminal alcohol may be oxidized in the presence of the oxidizing agent. For example oxidation of aldehyde group results in the formation of gluconic acid and the oxidation of terminal hydroxyl group results in the formation of glucuronic acid.
IV.) Reduction reaction
The aldehyde or carbonyl group of monosaccharides are reduced to their corresponding alcohols by treating them with reducing agents like sodium amalgam.
V.) Dehydration reaction
⇒ when carbohydrates treated with concentrated sulphuric acid they undergo dehydration with the elimination of 3 water molecules hexoses gives hydroxymethylfurfural while pentoses give furfurals on dehydration
⇒ The farfural can be condensed with phenolic compounds like Alpha naphthol to form color product ( purple ring) this is the chemical basis of molisch test.
VI.) Osazone (crystal) formation
⇒ When monosaccharides react with phenylhydrazine then it results in the formation of osazones.
⇒ Glucose, Fructose, and mannose give the needle-shaped osazones.
⇒ Maltose forms sunflower type crystals and Lactose forms cotton balls shaped osazones.
VII.) Formation of esters
⇒ The alcoholic group of monosaccharides maybe esterified esterification of Carbohydrates with phosphoric acid is a common reaction in carbohydrates metabolism, for example, Glucose-6-phosphate & Glucose-1-phosphate is formed by esterification of glucose.
VIII.) Mutarotation
In aqueous solution, the α- & β- anomers of monosaccharides convert into each other by a process called muta-rotation.
IX.) Glycoside formation
⇒ They are formed when hydroxyl group of an anomeric carbon of Carbohydrates react with the hydroxyl group of another carbohydrate, the Bond so formed Alpha 1→4 or Beta 1→4 is known as glycosidic Bond.

Hi, I’m the Founder and Developer of Paramedics World, a blog truly devoted to Paramedics. I am a Medical Lab Tech, a Web Developer and Bibliophiliac. My greatest hobby is to teach and motivate other peoples to do whatever they wanna do in life.
Superb notes
Thanks
Thanks, for your good work, I am biochemist from Nigeria