Staining of the tissue sections is carried out to study the Morphological and relationship of tissues and their constituent cells. Various types of dyes either Natural or Synthetic, are used to prepare the staining solutions. Stains are frequently used in the Histopathology & Cytopathology laboratory to highlight the structures of biological tissues mainly with the aid of Microscopes.
Stains may be used to define & examine the bulk tissues, Cell population or Organelles within the individual cells. In histopathology laboratory, the Hematoxylin and Eosin stain is referred as the Routine Stain as it can be used to stain any tissue specimen to reveal the underlying tissue structures and conditions. In the Histology and Histopathology laboratory, all the specimens are initially stained with the Hematoxylin & Eosin stain and Special stains are only done in order to obtain the additional or more detailed information of the tissues.
HEMATOXYLIN & EOSIN STAINS…..
Hematoxylin is extracted from the heartwood of the logwood tree Hematoxylum campechianum, when oxidized, it forms the Hematein, a compound that forms the strongly colored complexes with certain metal ions, mainly Iron (Fe) and Aluminium (Al) salts, Metal-Hematein complexes are used to stain the cell nuclei prior to examination under the microscope.
Eosin is most suitable combination used with the Hematoxylin for the routine staining purpose. Eosin belongs to Xanthene group & it is anionic dye by nature. Anionic dyes are important for staining cytoplasm & extracellular structures. Various types of Eosin stains are available, like Pink, Red, Orange, and Yellow. Eosin Yellow (Y) is the most commonly used stain in histopathology laboratory.
PREPARATION OF HEMATOXYLIN STAIN…..
Harris Hematoxylin Stain –
- Hematoxylin ⇒ 1 gm
- 95% (v/v) Ethanol ⇒ 10 ml
- Ammonium or Potassium Alum ⇒ 20 gm
- Mercuric Oxide ⇒ 0.5 gm
- Distilled Water ⇒ 200ml
Dissolve the 1.0 gm of Hematoxylin in 10 ml of Ethanol (95%) in a mortar with a pestle and 20 gm of Ammonium or Potassium Alum in 200 ml of warm distilled water. Mix the Two solutions while warm and quickly bring it to boil with constant stirring. Now add 0.5 gm of Mercuric oxide. immediately remove the content from the flame and cool it as quickly as possible using running tap water. Filter the content and Store in a brown or Amber colored bottle. The stain is stable at room temperature (20°C – 30°C) for several months.
PREPARATION OF EOSIN STAIN…..
Eosin Y Stain –
- Eosin Y ⇒ 1 gm
- Distilled water ⇒ 80 ml
- Ethanol 95% (v/v) ⇒ 320 ml
- Glacial Acetic Acid ⇒ 0.4 ml
Dissolve the 1.0 gm of Yellow eosin in about 80 ml of distilled water. To this, add 320 ml of 95% (v/v) ethanol. Add 0.4 ml of Glacial Acetic acid. Mix well. This solution is stable at room temperature (20°C – 30°C) for several months.
PRINCIPLE OF HEMATOXYLIN & EOSIN STAINING
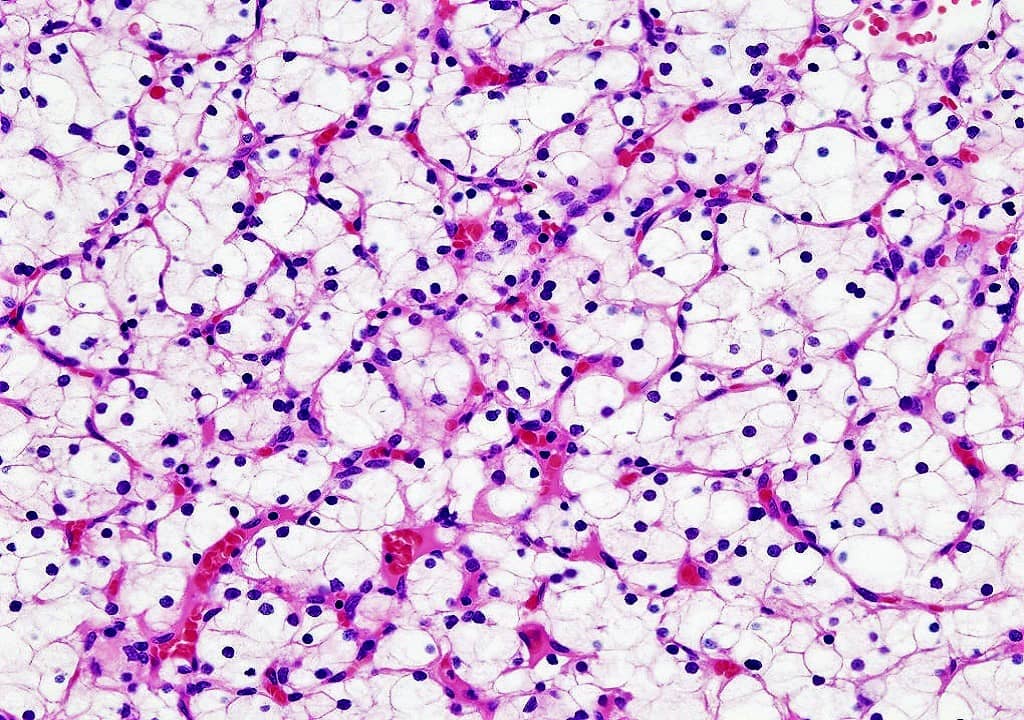
Hematoxylin and Eosin are the principle stains used in the histopathology laboratory for the demonstration of the nucleus and the cytoplasmic inclusions. Hematoxylin is a Basic dye that stains the acidic components of the cell i.e. the nucleus whereas Eosin is the Acidic dye that stains the Basic components of the cell i.e Cytoplasm. Alum acts as a mordant and Hematoxylin containing alum stains the nucleus light blue which turns red in the presence of acid and dark blue in the presence of alkali. The cell differentiation is achieved by treating the tissues with the acid solution and then with Alkali solution. The counterstaining is performed by using eosin solution which imparts the pink color to the cytoplasm.
REQUIREMENTS FOR HEMATOXYLIN & EOSIN STAINING
- Coplin Jars
- Dropping bottles (50 ml)
- Coverslips
- Specimen slides
- Slide washing tray
- DPX or other Suitable mounting media
- Microscope
Reagents
- Harris Hematoxylin stain Solution
- Eosin Y stain solution
- 0.5 % (v/v) Hydrochloric acid
- Diluted ammonia water (1.5 ml of 28% (v/v) ammonia solution in 1500 ml of distilled water)
PROCEDURE OF HEMATOXYLIN & EOSIN STAINING
⇒ Deparaffinize the section – Warm the Slides on the slide warming table for 5-10 minutes or flame the slides on a burner and then place it in the Xylene for 3 to 4 minutes. Repeat the xylene treatment 2-3 times with agitation.
⇒ Rehydrate the section by passing it through the graded alcohol in decreasing order as Absolute alcohol >> 90% Ethanol >> 80% Ethanol >> 70% Ethanol >> 50% Ethanol >> 30% Ethanol. Place the section for 1-2 minutes in each of these alcohol solutions. Wash in tap water and rinse in the distilled water. Drain the sections well before staining with dyes.
⇒ Dip the Slides in the Coplin jar Containing the Hematoxylin stain or apply the Hematoxylin stain on the tissue sections on the slides for 3-5 minutes. Wash the slides in running tap water.
⇒ After washing, dip the slides in and out of the 0.5% (v/v) Hydrochloric acid (HCl). (Check the Differentiation by using a microscope. The Nuclei should appear dark purple or Reddish blue and the tissues appears pale). Rinse the slides in Tap water for 1-2 minutes.
⇒ Now, Dip the Slides several times in the diluted ammonia water. The section should appear as blue colored. Rinse the slides in tap water.
⇒ Dehydrate the specimen using graded alcohol in ascending order as 50% Ethanol >> 70% Ethanol >> 90% Ethanol >> 95% Ethanol. Place the specimen for 30 seconds to 1 minute in each of these alcohol solutions and finally rinse with Absolute Ethanol.
⇒ Dip the slides in Eosin solution for 30 seconds to 1 minute. Rinse the Slides with Absolute alcohol.
⇒ Place the slides two times in Xylene solution for 30 seconds to 1 minute each.
⇒ Drain the excess Xylene and mount with the DPX and place the coverslip on it. Let it dry and observe under the microscope.
RESULT INTERPRETATION
Cell nuclei – Blue Color
Erythrocytes – Red color
Muscles, Connective tissues, Cytoplasm – Varying Shades of Pink

Hi, I’m the Founder and Developer of Paramedics World, a blog truly devoted to Paramedics. I am a Medical Lab Tech, a Web Developer and Bibliophiliac. My greatest hobby is to teach and motivate other peoples to do whatever they wanna do in life.
sir,
i have face few problems in h&E staining. please you send me whole procedure H&E staining with making the stain by powder. thanks
Hello Rajkumar,
Here is the H & E Protocol….
If you have any other queries… don’t forget to mention in comments.
Thank You.
Good morning Sir,
Please can you send me the full protocol of Immunohystochemistry staining?
Thank you
Great work done sir , very very use full in B.Sc MLT study Thanks I am using notes and procedures from your web.
Thank Q sir
Most Welcome 🙂
Why there is requirement of rehydrate and dehydrate steps
can you please elaborate this aspect more ??????
Hematoxylin as a stain has many applications. Discuss
Hello. I have a little issue with the H&E staining. The issue stain seems to overpower and overshadow the Hematoxylin stain even though it is stained in Hematoxylin for 10mins and Eosin for 5 seconds. How do i tone down the eosin dye or concentrate the hematoxylin dye. Thanks